
Many surface disinfectants, especially in an aerosolized form, can be harsh not only for us, but also for our pets’ skin and mucous membranes. And many topical disinfectants and antimicrobials for wound cleaning only come in small quantities, designed to target small, affected areas. But what if we told you that, with minimal effort, ingredients, and investment, you could make one of the most powerful antimicrobial cleaners known to humans by yourself, at home? It’s true! Let’s talk about it and then go step-by-step for producing your own hypochlorous acid, or HOCl easily.
What is Hypochlorous Acid (HOCl)?
Hypochlorous acid is a weak acid naturally produced by white blood cells in humans and animals as part of the immune response. It has powerful antimicrobial properties, making it effective in killing bacteria, viruses, and fungi. Commercially, HOCl is used in disinfectants, wound care products, and skin cleansers.
Positive Effects for Humans and Pets
- Antimicrobial Properties
- Humans: HOCl is effective against a broad spectrum of pathogens, making it ideal for disinfecting skin, wounds, and surfaces without harsh chemicals.
- Pets: Used to clean wounds, hot spots, ear infections, and skin irritations safely.
- Non-Toxic and Gentle
- Unlike bleach or alcohol-based disinfectants, HOCl is non-irritating to skin and mucous membranes, making it safe for frequent use on both humans and animals.
- Wound Healing Support
- Humans: Promotes faster healing by reducing bacterial load without damaging healthy tissue.
- Pets: Reduces inflammation and helps manage minor cuts, scrapes, and post-surgical sites.
- Eye and Ear Care
- Safe for cleaning around the eyes and ears, especially for pets prone to infections like dogs and cats.
- Anti-fungal Properties
- Hypochlorous acid (HOCl) is effective against fungi, including various types that cause infections in both humans and pets. Its broad-spectrum antimicrobial properties make it useful for managing fungal-related issues.
- HOCl disrupts the cell walls of fungi, leading to the breakdown of their structure and eventual death. It’s effective against yeasts, molds, and dermatophytes (the fungi responsible for skin infections like ringworm).
- Environmental Safety
- Biodegradable and breaks down into water and salt, posing no environmental risks.
Hypochlorous Performance and Kill List
Let’s look at some of the common pathogens that HOCl is very effective at killing.
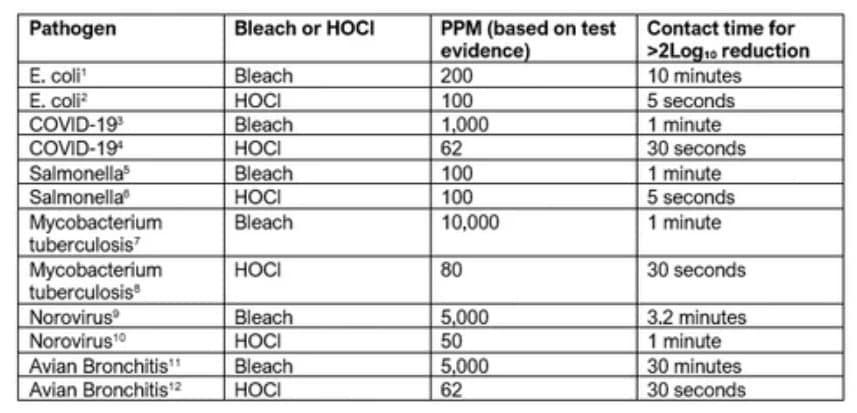
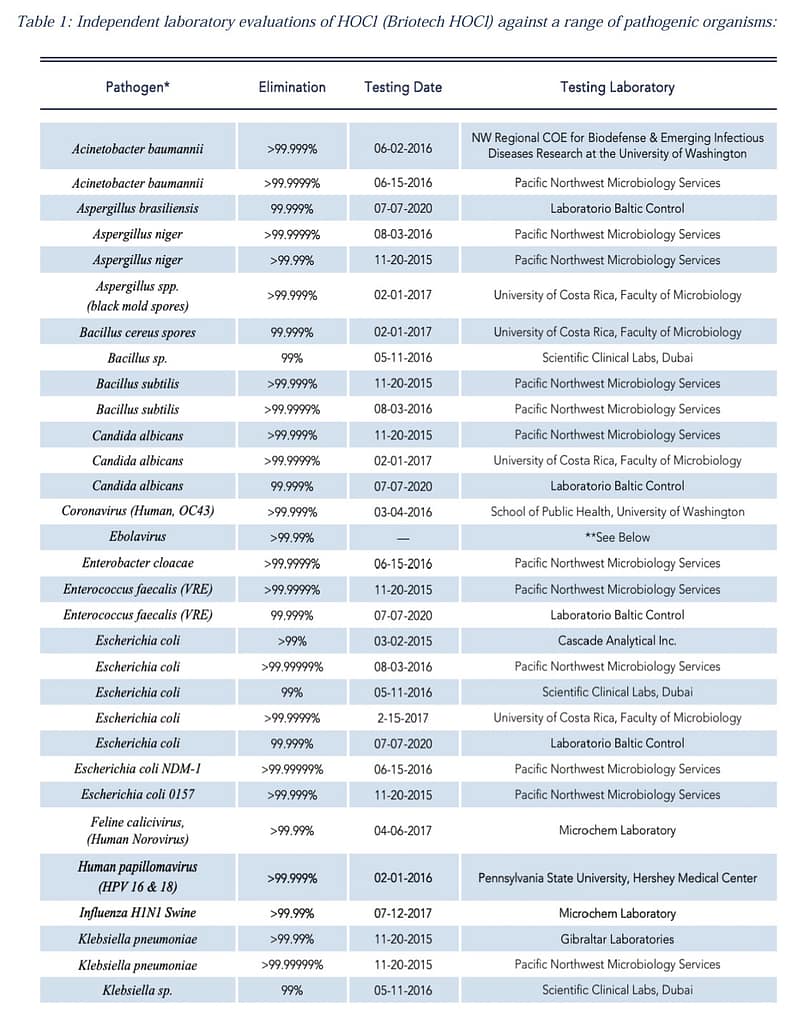
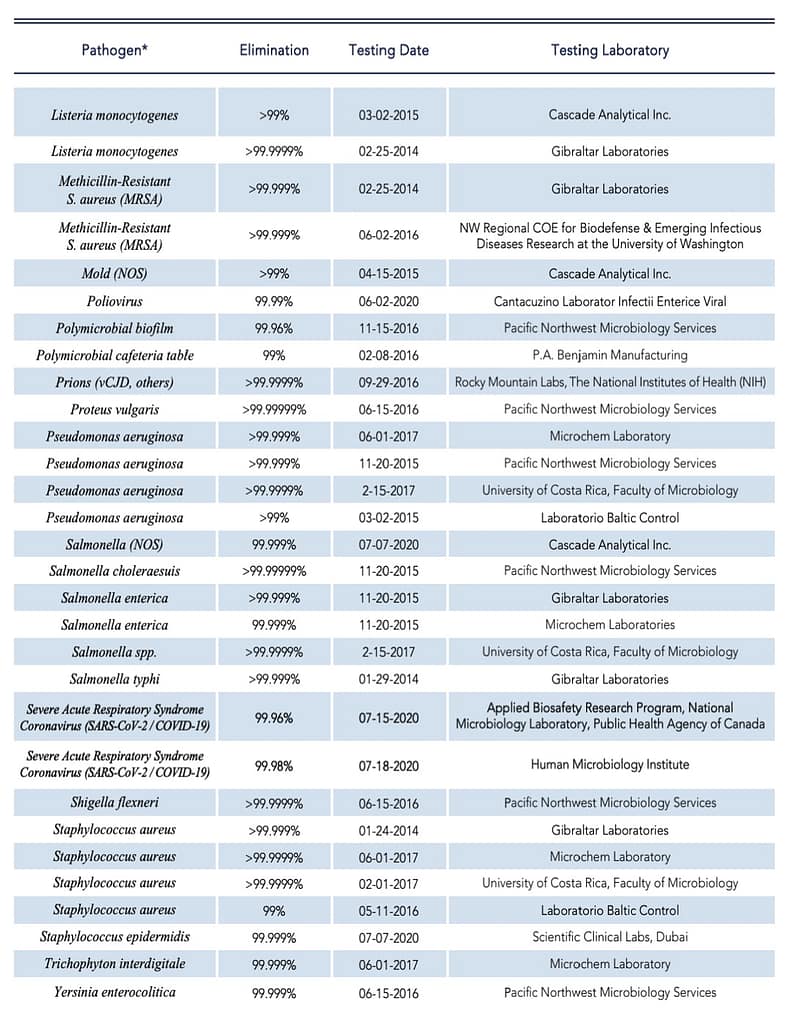
Impressed? We certainly are! For more links and information about the pathogen killing power of HOCl, we’ll provide those for you below.
Ready to Make Hypochlorous Acid?
HOCl requires two basic ingredients:
- Salt
- Water
Salt contains chlorine within it, but the HOCl requires an electrical current to run through a salt and water mixture for a specified amount of time in order to extract and “activate” the HOCl particle.
Fortunately, you don’t have to risk electrocution to make this amazing pathogen killer! You can either use a portable electrolysis machine or, the easiest way possible, a pitcher that is designed to automatically create HOCl using a water and salt mixture.

But wait, there’s more! (and it’s important)
All of these devices suggest the addition of vinegar to the mixture to make it more effective. The reason why is worth digging into.
Why the addition of vinegar?
Vinegar is an acidic substance, as we know. But why is it always recommended to add a little bit of vinegar to our salt + water mixture to really get the best effectiveness from our home-made hypochlorous acid? Now, you can just do that, without worrying much about it (just trust us and the chemists)! But, if you want to know why it’s incredibly helpful to add vinegar to our base misture, read on.
What is pH and why is it important?
pH is the “potential of Hydrogen” and in our discussion here, the important thing to understand is that pH is the measure of acidity or alkalinity of a substance. And when we are talking about HOCl, pH is very important.
The following diagram shows where certain common substances live on the pH scale:
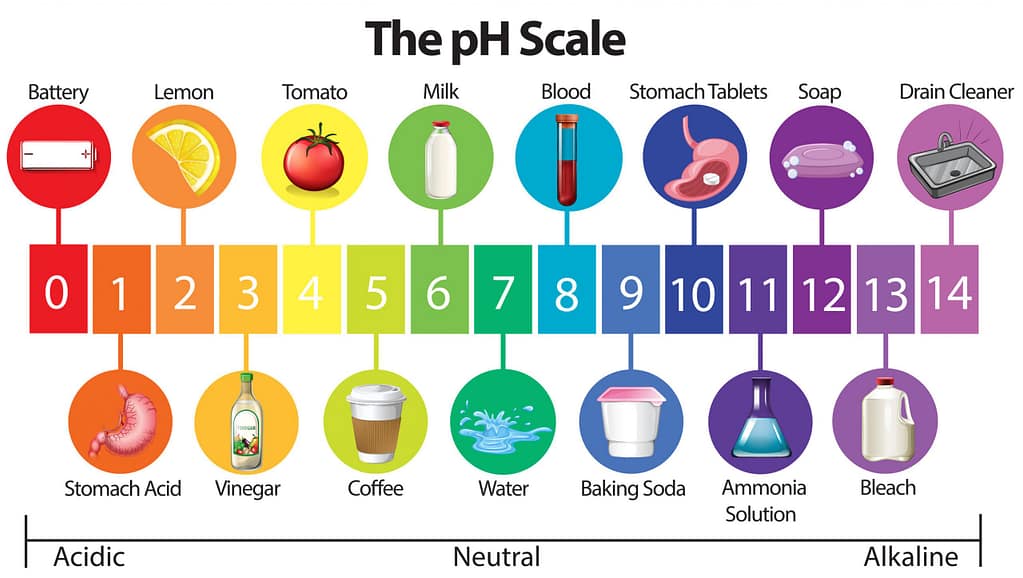
What we can glean (we said “glean”) from this pH chart is that low pH means ACID, whereas high pH means ALKALINE. And when we push an electric current through our water + salt (+ vinegar) mixture, the level of pH is going to determine how much HOCl particles exist in our mixture, and that is linked to the efficacy of our home-made disinfectant.
Let’s look at this chart which shows where HOCl is most active.
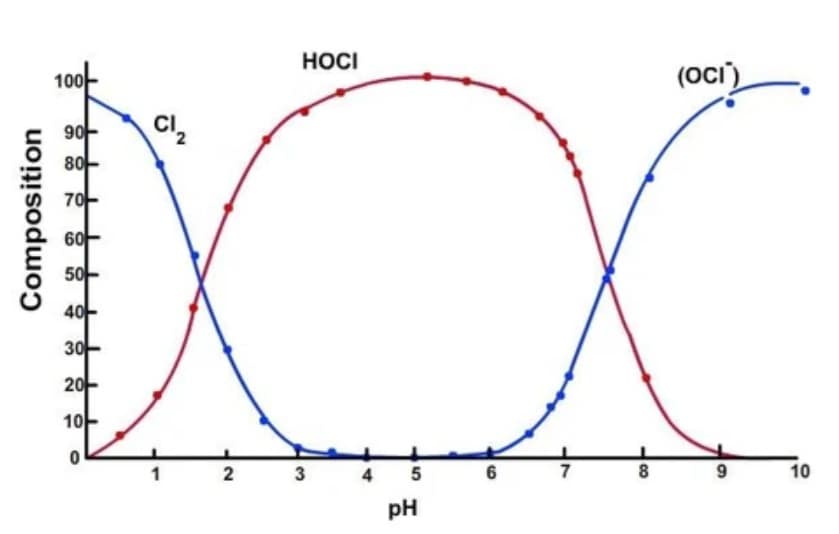
This chart shows that HOCl is most active in a pH solution between 3 and 7 on the pH scale. And since we know that the time we add an electric current to our basic water + salt (+ vinegar) mixture affects the pH (the more time under the current, the higher the outcome on the pH scale), how do we get to the ultimate acidity to produce the most HOCl particles in our mixture?
Without the addition of vinegar, most electrolyzed solutions after 10 minutes or end up at around 7 on the scale. That works, but we can do better.
Where does HOCl live on the pH scale?
Hypochlorous Acid lives at its most active at pH scale 5! But water and salt in its native state already starts at a 6 and then continues up from there.
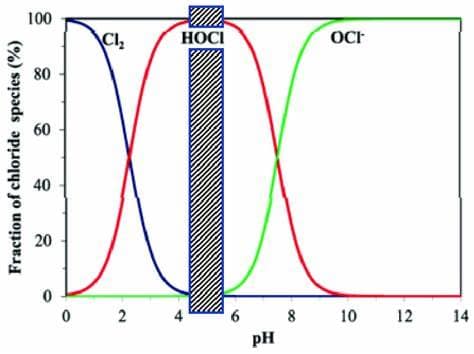
There it is. We are looking for a 5. How does vinegar help us?
White vinegar, distilled at 5%, exists as a 2-3 on the pH scale. Adding just a little bit to our mixture (the recipe and ratios to follow) pushes us back towards ACID on the scale, giving us the perfect playing field for a slightly more acidic mixture, resulting in achieving a 5. That’s what we want.
Hypochlorous Acid Recipe
10 fluid oz / 300 ml filtered water
1 tsp salt (fine, kosher sea salt if possible)
1 ml vinegar – white 5% distilled
Electrolysis for a specified time – typically 10 minutes ( with either a pitcher or a travel electrolysis device)
Testing your mixture
The thing about HOCl, it really doesn’t have an odor (very slight, maybe best defined as “clean”), and the pH is close to basic water + salt. In order to verify that we have HOCl, we should use test strips to see what we’ve brewed.
We want our pH strip (after dipping into our mixture) to be a 5, so close to this color on this particular brand of testing strip:

To verify that we have something that contains chlorine (good, healthy chlorine that doesn’t smell or bleach your hair), check your chlorine testing strips to see if your mixture contains chlorine matching the 200ppm (that’s parts per million) color:

Once you verify that you have a slightly acidic mixture (a 5 on the pH scale) and that your solution has chlorine, you have done it! You have successfully made Hypochlorous Acid at home!
How to Store Homemade Hypochlorous Acid
Once you have made your “liquid gold of disinfection”, proper storage is crucial to make your mixture last as long as possible. HOCl should be stored in a light-proof container (we like to use it in a spray bottle) in a temperature controlled area. Make sure to test your mixture regularly after 3-4 weeks to ensure that it is still maintaining its efficacy. And don’t forget to label your bottle clearly with what it contains and, ideally, the date the last batch was made.
Things to Watch Out for with the Misuse of Hypochlorous Acid
Is Hypochlorous Acid a little too good to be true? It’s better. But, HOCl is a weak acid, so there are some precautions that we should be aware of to make sure we use it as safely as possible.
- Skin Irritation (in Rare Cases)
- Overuse or high concentrations may cause mild skin dryness or irritation, particularly in sensitive individuals or pets.
- Respiratory Irritation (Aerosolized Form)
- Inhalation of misted HOCl in poorly ventilated areas might irritate the respiratory tract in both humans and pets.
- Instability
- HOCl is unstable and can lose its effectiveness quickly if not stored properly (exposure to light, heat, or air).
- Potential Eye Irritation (at High Concentrations)
- While generally safe, direct contact with concentrated solutions may cause temporary discomfort in the eyes.
- Misuse Risks
- Confusion with stronger chlorine-based disinfectants (like bleach) could lead to misuse, potentially causing harm.
- Staining of Metal Surfaces
- Because HOCl is a weak acid, it can affect the appearance of certain metals. For instance, If using HOCl to disinfect a stainless steel tub, you should rinse thoroughly after a few minutes to ensure that the acid does not affect the look or color of the metal.
Are you excited about making Hypochlorous Acid yourself?
You should be! Hypochlorous acid has so many benefits that once you have it in your home, you will find yourself using it for many of its amazing uses.
Want to see a step by step video tutorial on making hypochlorous acid? We’ve got you!
Any questions or comments? Let us know what you think and please share your thoughts and experiences with HOCl!
#petgroomingpost








Leave a Reply